Introduction Sickle cell disease (SCD) is a hereditary hemoglobinopathy characterized by chronic hemolytic anemia, devastating ischemic pain crises and chronic organ damage, resulting in shortened lifespan. Ethnicity and race are often mentioned in SCD research. Possibly, since SCD is particularly prevalent amongst people who originate from sub-Saharan Africa.
In research studies, ethno racial categories (ERCs) are frequently considered confounding variables. However, these ERCs are often large aggregate categories and include individuals with a diversity in phenotype, exposures and genetic make-up. Recent journal submission guidelines urge researchers, when applicable, to declare the relevance of the use of ERCs to the study as well as the modality through which an ERC is determined (e.g. self-identification, country of birth, visual identification by the observer). Providing such detailed information is important because true confounders with a specific feature might distort study results. However, when operationalizing ERCs in SCD research, there exists a risk of reification of already marginalized populations as both a social and a biological group. Therefore, contextualization is essential when deciding to use ERCs in SCD research.
This study aims to provide an overview of how ethnicity and race are applied in SCD research.
Methods We systematically searched the literature to explore the current practice of ERCs in SCD research across world regions. Embase and Medline were systematically searched in November 2022 for all published articles on SCD published from 2011 onwards. Studies were considered eligible when cases defined as individuals with SCD and controls individuals without SCD. A total of 1,105 articles in SCD research were included. Of these articles, 1,085 were domestic studies and 20 international studies. Statistical tests and models implemented in R included chi-square tests and univariate linear regression.
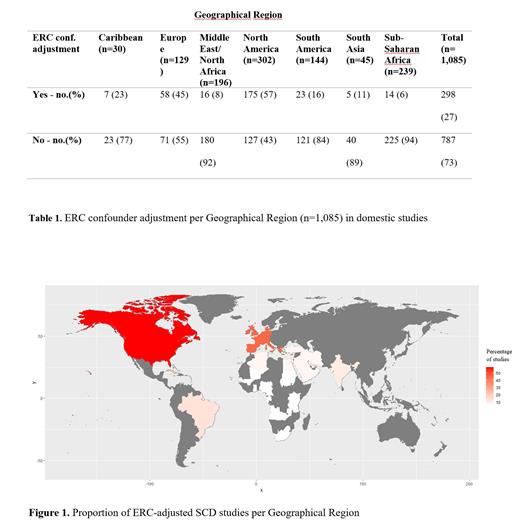
Results In 27% (298/1,085) of all studies, ERC confounder adjustment occurred. This methodological practice was dependent on geographical region the research subjects were sourced from (p<0.05), as made visible in Figure 1. In 175/302 (57%) of studies with a North American study population ERC confounder adjustment took place, as well as in 45% [58/129]) of the studies reporting on a European study population. In contrast, in 6% (14/239) of sub-Saharan African study populations, ERC confounder adjustment was performed (Table 1). Of the twenty international studies, six operationalized ERCs as confounders. In five publications, the control group was sourced from the same country as the SCD cases. One study used a healthy Congolese reference population as a control cohort whilst the SCD patients were from France. ERC confounder adjustment also varied with the biomedical discipline in which the article was published. Compared to Hematological journals, articles published in journals with categories “Medicine, General & Internal medicine” (OR=0.30, 95% CI -1.76 - -0.67, p<0.0001), were less likely to adjust for ERCs as confounders whereas articles in Ophthalmological journals (OR= 3.36, 95% CI 0.27 - 2.23, p=0.01) were more likely to do so. Of the studies that used ERC confounder adjustment, 80% (239/298) did not explain why this was relevant. Furthermore, in 70% (209/298) of these ERC-adjusted studies, it was unclear through which modality ERCs were determined. In the papers that described modalities, 49% (44/89) used ERCs from pre-existing databases, 18%(16/89) used sibling/relative recruitment, 30% (27/89) used self-reporting ERCs and 2% (2/89) used multiple ways to classify ethnicity or race. Moreover, in 28% (83/298) of the ERC-adjusted studies, it was not clear which specific categories were used.
Conclusion ERC confounder adjustment is a common occurrence in SCD case-control studies in the United States and Europe. Furthermore, contrary to current author guideline recommendations, reporting on ethnicity and race in SCD studies is frequently inconsistent and incomplete. This confuses the possible relevance of ERCs to the study and inadvertently reinforces reification of these constructs. Moving forward, we recommend the promotion of global guidelines around the use of ERCs in case-control studies to stimulate further research into uncovering specific relevant mechanisms that create differences in health outcomes.
Disclosures
Kidane:CSL Behring: Research Funding. Rab:Pfizer: Research Funding; Agios Pharmaceuticals: Research Funding. Rijneveld:BMS: Honoraria; Servier: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal